Hard water can cause white spots or scale deposits on sinks, fixtures and dishes. Yellowish or gray laundry is also a sign that a house is being supplied with hard water. A hard water test kit can be used to confirm the presence of hard water and the concentration of the minerals that cause water hardness.
Almost 90% of households have hard water, which is water high in dissolved minerals, largely calcium and magnesium. Water from most domestic wells and from public utilities is likely to contain these dissolved minerals. In fact, municipalities count on water hardness to provide a necessary daily intake of calcium and magnesium to residents.
In this article, we’ll discuss hard water, its signs around the home and some of the consequences. We also give recommendations on the best treatments to reduce hardness, including the best water softeners in the market.
- Hard water can have a metallic taste and cause dry skin and hair.
- The minerals in hard water cause scale deposits to build-up on fixtures, and inside pipes and heating appliances, such as coffee makers and water heaters.
- A hard water test kit can be used to collect a water sample and send to a certified laboratory to determine the level of water hardness in your home.
Signs your house has hard water
Hard water is water containing high amounts of dissolved calcium and magnesium ions. It also often contains some aluminum, barium, strontium, iron, zinc, silica and manganese.
Calcium and magnesium enters our groundwater as rain water moves through soil and rock (such as limestone or dolomite) and dissolves the calcium carbonate minerals.
This is why surface water, such as in rivers and lakes, generally has lower levels of hardness, while groundwater can have quite high levels.
Hard water is especially prevalent in the east-central and western United States, due to the area’s geology. The majority of the country draws their tap water from carbonate aquifers or aquifers with high concentrations of dissolved solids (including calcium and magnesium), except for coastal regions where water tends to be softer.
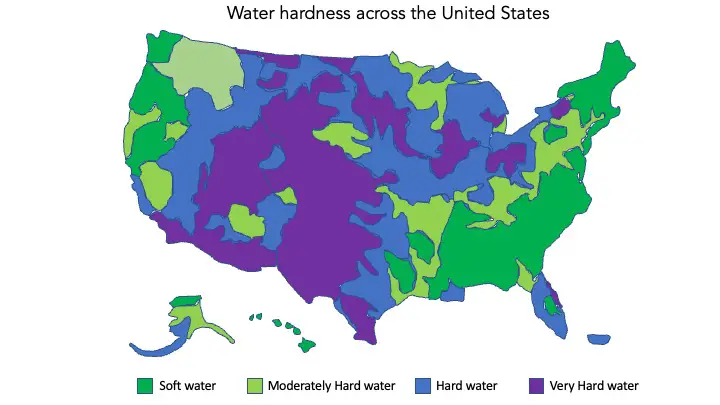
Carbonate aquifers contain compounds such as carbonate, bicarbonate, hydroxide and sometimes borate, silicate and phosphate.
- When calcium or magnesium is combined with these compounds, which are alkaline, they produce carbonate hardness. This is also known as temporary hardness because it can be removed by boiling the water.
- Non-carbonate hardness, formed when calcium and magnesium combine with non-alkaline compounds (such as sulfate), produces a type of hardness that is known as permanent, which cannot be broken down with heat.
The levels of hardness (carbonate and noncarbonate) in water are measured in milligrams per liter (mg/L) and described in 4 classes:
- Soft water: 0 to 60 mg/L
- Moderately hard water: 61 to 120 mg/L
- Hard water : 121 to 180 mg/L
- Very hard water: >180 mg/L
1. Aesthetic and Operational Effects
Water hardness has some obvious aesthetic and operational signs that you can easily spot:
- Metallic taste and sometimes odor.
- Dry skin and hair.
- Soap scum and scale deposits on fixtures, bathtubs and sinks.
- Low water pressure due to clogged pipes.
- Hard deposits in heating appliances (e.g., water heater, coffee maker)
Metallic taste and (sometimes) odor.
Hard water has a metallic taste because of the calcium and magnesium compounds. It can also create a slightly acidic, bitter or sour taste depending on the compounds.
For example calcium chloride is more likely to make water taste acidic or bitter, while water with calcium gluconate is more likely to taste sour or metallic.
Dry skin and hair.
The dissolved calcium and magnesium in hard water causes dryness in our skin and hair. Showering or bathing in hard water causes increasingly dry skin to lose more water by evaporation, and is known as transepidermal water loss.
Hard water also causes skin irritation and can lead to atopic dermatitis, a condition of dry, itchy skin, inflammation and even lesions.
Soap scum and scale deposits on fixtures, bathtubs and sinks.
The calcium and magnesium also reacts with the fatty acids in soaps and detergents, producing an insoluble gelatinous scum that stops the soap from being able to foam.
Non-foaming soap is not just a simple annoyance, hard water also reduces the soaps ability to clean. More soap is needed to wash things and scum easily builds up in rings or spots around the bathtub.
Scale, or limescale, deposits also form from the minerals in hard water. These deposits are found in and on anything that is regularly wet, and then allowed to dry. Shower heads, faucets and sinks are some of the most common places to find scale deposits.
Oxidized ferrous iron is also often present in hard water, leaving reddish brown stains on washed fabrics and enameled surfaces.
Low water pressure due to clogged pipes.
Hard water also causes mineral build-ups or deposits in plumbing. This can clog pipes and reduce the performance and lifespan of appliances.
Overtime, these deposits can slow the flow of water and water pressure at the faucet. However, it’s important to note that using a water softener to remove water hardness can make the water more acidic and corrode pipes instead, potentially leaching lead into the water.
Hard deposits in heating appliances.
Hardened calcium carbonate deposits in heating appliances is similar to the mineral build-ups in pipes. However, heating the water causes the deposits to build quickly and can rapidly damage your water heater, coffee maker etc.
An easy solution to these hard water deposits is to run vinegar through your coffee machine (or other appliances), while your water heater should be flushed every year to 3 years depending on how hard your water is.
2. Test for water hardness
Aside from the noticeable signs around your home that you have hard water you can also test for it directly.
To determine the level of hardness in your tap water when it comes from a public source, you can contact your water utility to receive a report on the compounds present in your water. Or you can get a test kit to analyze the quality of your supply, whether you use public water or a domestic well.
The benefit of a test kit is that you will know how hard your water is e.g., moderately hard, hard, or very hard. This can help you decide how to best manage your water. People often wonder whether they need a water softener, but unless you have very hard water, most effects of moderately hard to hard can be managed in the home without one.
To test for water hardness at home, simply collect a sample and send it to a certified, independent laboratory for analysis. We recommend this test from Tap Score, they will send you everything you need and will have your detailed results back to you within 5 days.
A cheaper option is to buy water hardness test strips from Amazon – there are tons available, but this one covers the full water hardness range and is adequate for home use. Just remember these will expire and may give less accurate results than the laboratory test (mentioned above) especially if the strips are not stored properly or are exposed to the air (and moisture) for long periods.
Health related effects of hard water
According to the Department of Agriculture (USDA), residents living in hard water areas who drink 2 liters of water a day ingest about 61 mg of calcium and 19 mg of magnesium from their water. This is about equal to 5-6% of the daily recommended intake of each mineral.
The World Health Organization has declared that hard water has no known adverse health effects. In fact, the WHO suggests that it is actually a source of necessary dietary minerals.
Calcium is a critical component of bones, and has many positive effects on the body, such as prevention of serious life-threatening and painful ailments like osteoporosis, kidney stones, hypertension, stroke, obesity, and coronary artery disease.
Healthy individuals are also naturally protected from any excess intake of calcium by their metabolism. This means that consuming water with high amounts of calcium is not harmful.
Magnesium also has positive health effects. However, it is believed that the increased intake of magnesium salts can possibly have a negative impact on gut health. Inadequate amounts of magnesium in the body increase the risks for some forms of health problems, such as hypertension, cardiac arrhythmia, coronary heart disease, and diabetes mellitus.
Several epidemiological investigations have found a relation between risk for cardiovascular disease, growth retardation, reproductive failure, and other health problems and hardness of drinking water, in particular its content of magnesium and calcium. The acidity of the water may magnify the health effects of water hardness, as it affects the ability to reabsorb calcium and magnesium in the intestinal tract.
However, the evidence is constantly being debated and it has been pointed out that these studies did not find a causal link – this means the patients were drinking hard water and had health problems, but not that the hard water actually caused the health problems.
At the same time, other studies have shown that people who drink greater amounts of soft water have much higher incidences of heart disease, as well as higher blood pressure and cholesterol levels, and faster heart rates than those who drink mostly hard water.
This is probably caused by the sodium that is introduced into the water to remove the calcium and magnesium.
How to treat hard water at home
Hard water can affect your laundry, dishes, fixtures, as well as your wallet, your skin and your hair. For this reason, many industries and households use water softening treatments.
Home water softeners use natural or artificial zeolite minerals to precipitate the calcium as carbonate and the magnesium as hydroxide, and exchange the calcium salts with sodium carbonate or potassium ions. This is called ion exchange technology, which can also be designed to remove iron and manganese, heavy metals, some radioactivity, nitrates, arsenic, chromium, selenium, and sulfate in the water.
How to treat hard water without a water softener
Water hardness caused by calcium carbonate, known as temporary hardness, can be removed by boiling the water. Permanent hardness cannot be treated by heat but can be reduced with a reverse osmosis system.
The high pressure of reverse osmosis technology forces the water through a semipermeable membrane that absorbs the larger ions responsible for the water’s hardness. The disadvantage of this filtration method is that it generally produces high water waste and the hard water ‘fouls’ the membrane fairly quickly.
A practical alternative are water pitchers, which in some cases are effective in softening hard water. Water Purification guide has more information on this topic available here.
